ISO/IEC 17025
Accreditation from British Accreditation Registrars (BAR-UK)
Raise your standards for testing and calibration by gaining ISO/IEC 17025 Accreditation for your laboratories.
Different testing laboratories often produce somewhat different test results for a given same sample due to a variation in staff competency, equipment or tool used for testing, technologies they operate in the labs, and Standard Operating Procedures (SOPs) followed. These variations are due to the use of uncalibrated and unmaintained instruments to updated technologies and techniques, and the competence level of technicians conducting a particular test. Another reason for this variation is often done by manipulating sampling procedures and test results. Well, there can be countless factors and reasons contributing to the inconsistency and inaccuracy of test results and the decisions derived from them! So, to maintain the consistency in the report and test performed; ISO/IEC 17025 accreditation came into force to demonstrate worldwide accepted accurate results.
ISO/IEC 17025 Accreditation is a company-level accreditation published by International Organization for Standardization in cooperation with International Electrotechnical Commission. ISO/IEC 17025 provides “General requirements for the competence of testing and calibration laboratories”. The main motto of this accreditation is to offer quality and improve the processes within laboratories. The standard contains two main sections: 1. Management requirements and, 2. Technical requirements. Management requirements are primarily related to the operation and effectiveness of the quality management system within the laboratory and technical requirements are primarily related to the competence of staff and calibration of equipment and tools used in the laboratories. The standard also gives requirements related to quality management such as document control and corrective action required to implement if the case arises.
ISO 17025:2017 is the main ISO standard used by third-party laboratories to get their lab’s international identity. In most cases, suppliers and regulators will not accept their test results from a laboratory that does not hold accreditation. Thus, ISO/IEC 17025 is by far for the laboratory to establish competence and quality for their testing and calibration. It also facilitates cooperation between testing laboratories and other bodies by establishing wider acceptance of results. Test reports and Certificates of Analysis (CoA) can be accepted by organizations present in different countries without the need for further testing if your laboratory is accreditated by ISO/IEC 17025 standard, which leads to better international trade—quality as per new technology and techniques to cater to customer requirements and needs.
ISO 13485 requirements cover-up all the Quality Policy, Quality Objectives, and Quality Manual for implementing the Quality Management System. Adopting ISO 13485 provides a practical foundation for manufacturers to address the EU Medical Device Directive (MDD), the EU Medical Device Regulation (MDR), and other regulations, as well as demonstrating a commitment to the safety and quality of medical devices for their users.
A Certification Body applying for ISO 13485 accreditation must conform to ISO/IEC 17021 and other additional International requirements as detailed in Specific Requirements for Accreditation for QMS-MD Scheme.
Benefits of ISO/IEC 17025 Certification from British Accreditation Registrars (BAR-UK)
ISO/IEC 17025 is an international standard that requires continuous improvement and self-correction as per updates in technology. A laboratory that complies with ISO/IEC 17025 enjoys various benefits in the following ways such as:
- Enhances the reliability of test results
- Establish technical competency
- The efficiency of the laboratory increases
- Customer complaints are reduced
- Laboratory gains a strong competitive edge
- Operational expenditure is reduced
- Facilitates traceability of measurements and calibrations
- Ensures the accuracy of test results
- Use of updated technology method
- Maintains a record of test
Why is Laboratory Management Accreditation important for you?
Becoming certified against ISO/IEC 17025 demonstrates that you commit to implementing the requirements of compliance with the standard. As a certified professional, you will enable that your laboratories demonstrate and operate the standard competently, and can generate valid results accepted globally. Nowadays many organizations have started to offer contracts only to certified professionals and laboratories, as the majority of customers prefer health concerns. Therefore, ISO/IEC 17025 Certification helps you to receive services consequently, enabling you to maximize your earning potential.
What are the requirements for ISO/IEC 17025 Accreditation?
The process approach towards ISO/IEC 17025 Certification highlights the large number of activities that need to be accomplished for acquiring a measurement result. For any test you wish to get performed, you will find trusted labs to get accurate results. To acquire this, the starting point is to map the processes and activities related to the measurement test. Then, look at the risks and determine which controls should be put in place to them corrected to provide the customers accurate results. Some requirements of ISO/IEC 17025 Certification that the lab need to perform are:
- Management of personnel competency(Well qualified and capable to perform the task)
- Establish document control of procedures for performing a particular task
- Determine effective technical records
- Provide suitable facilities and conditions while performing tests
- Updated suitable calibrated equipment and tools
- Usage of Certified reference materials
- Hire suitable reagents
- Follow suitable test methods and procedure
- Use validated methods
- Correct handling of samples for an accurate result
Apart from these main requirements other requirements include improvement in the work, making appropriate plans and policies, Customer feedback, Documentation, Corrective actions, Internal audit, and maintaining confidentiality.
Steps for ISO 17025 Accreditation
Now obtaining ISO 17025 is a cup of tea with these easy steps.
- Get Knowledge about ISO 17025:2017.
- Perform a Gap Analysis to study your drawbacks.
- Plan your project to overcome your drawbacks.
- Train your employees and personnel about the standard.
- Documentation.
-
- QMS Manual
- QMS Processes and Procedures
- Methods
- Forms & records
- Inter-Lab Comparison data and calculated z-score for each test parameter
- Measured Uncertainty values for each test parameter
Guidance on conducting risk/opportunity assessment.
Internal Audit by Your consultant.
External Audit by Certifying Body.
Obtaining Certification of Accreditation.
British Accreditation Registrars’ Accreditation for Inspection Agencies”
- Demonstrates compliance with ISO/IEC Standard 17025.
- BAR offers prompt, personal service, including rapid scheduling of assessments to meet the needs of inspection agencies.
- Accreditation serves as an internationally recognized “stamp of approval” for industry and regulators.
- Accreditation increases the recognition and acceptance of inspection reports across domestic and national borders.
- Accreditation helps to reduce costs for manufacturers and exporters by reducing or eliminating the need for re-inspecting in another economy.
LABORATORY ACCREDITATION- ISO 17025:2017
The laboratory accreditation process begins with a request for accreditation. BAR-UK provides laboratory accreditation to ISO/IEC 17025 and multiple standards in many industry-specific programs.
BAR-UK believes in a partnership approach to laboratory accreditation assessments. Throughout the laboratory accreditation process, we focus on customer needs while ensuring all ISO/IEC 17025 laboratory accreditation requirements are met. We’ve been in business, having recognized the need for accreditation bodies to oversee the work of third-party conformity assessment bodies and thus provide credibility and confidence for those who rely on tests, calibration results, and certification.
Our technically skilled and knowledgeable assessors know how to engage in a courteous, congenial, and meaningful manner. This is how working with BAR-UK makes the difference. The way we serve our customers sets us apart from other accreditation bodies.
Accreditation to ISO/IEC 17025 plays an important role in supporting the provision of accurate and reliable results from laboratory testing, calibration, sampling, and measurement services across many sectors.
The technical competence of a laboratory depends on a number of factors including:
- The qualifications, training, and experience of the staff
- The right equipment – properly calibrated and maintained
- Adequate quality assurance procedures
- Proper sampling practices
- Appropriate testing procedures
- Valid test methods
- Traceability of measurements to national standards
- Accurate recording and reporting procedures
- Suitable testing facilities
How to apply
Complete the application form relevant to your application – this will include details of what to submit, including key policy and procedure documents and proof of legal status, to ensure swift processing.
Please download and complete the relevant application form(s) to apply for accreditation here.
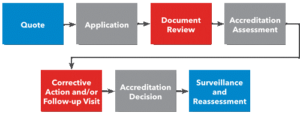
Certification Bodies interested to apply for Accreditation for ISO 13485 can send an email to info@bar-registrars.org demanding for following Application documents.
- Accreditation Application for Quality Management System for Medical Devices
- General Criteria for Accreditation Requirements
- General Criteria for Conditions for the Use of BAR Accreditation Symbol
- Applicable Fees
- Documentation Checklist as per QMS-MD
- BAR Transition Policy for ISO 17021-1:2015
Accreditation Criteria :
- ISO 17021 – Requirements for Bodies providing audit and certification of management systems.
- IAF MD 1:2007 – Certification of Multiple Sites Based on Sampling.
- IAF MD 2:2007 – Transfer of Accredited Certification of Management Systems.
- IAF MD 3:2008 – Advanced Surveillance and Recertification Procedures.
- IAF MD 4:2008 – Use of Computer Assisted Auditing Techniques for Accredited Certification of Management Systems.
- IAF MD 5:2013 – IAF Mandatory document for duration of QMS & EMS Audits.
- IAF MD 11:2013 – IAF Mandatory Document for the application of ISO 17021 for audits of Integrated Management Systems.



