CE MARKING
Accreditation for CE Marking (Conformitè Europëenne)
British Accreditation Registrars accredits Certification Bodies for compliance against CE Marking through verification of their existing ISO/IEC 17021 and ISO/IEC 17065:2012system. The accreditation is done against Scheme No- 50509.
CE Marking Accreditation for a Certification body (CBs) helps them to audit and provide accreditated Certification to their Clients’ products.
CE Marking is defined as the European Union’s (EU) mandatory conformity marking that indicates a product has been assessed by the manufacturer and deemed to meet EU safety, health, and environmental protection requirements, since 1985. The presence of CE marking further indicates that the manufacturer’s declaration that his product meets the applicable requirements with EU Directives.
There is an EU requirement that products not in conformity with the provisions of the directives are not allowed to circulate in the territories of the member states. The importer and/or manufacturer must take steps to comply with safety provisions, produce the appropriate records, and decide on the necessary procedures to maintain production in conformity with directives.
A Certification Body applying for CE Marking accreditation must conform to ISO/IEC 17021 and ISO/IEC 17065:2012and other additional International requirements as detailed in Specific Requirements for Accreditation for CE Marking Scheme.
Benefits of Accreditated Certification of GMP Certification
- Businesses perceive accredited certification as providing value for money
- Exceeds minimum standards of quality as per stated EU Directives
- CE marked products can be traded in the EEA without restrictions
- Providing assurance for the quality
- Achieve International approval
- Deliver value-added outcomes
- Leading independent market research company
- Employers often require evidence that the Certificate that they have received is from an Accredited Body
- Enjoy Competitor Edge
British Accreditation Registrars accredits Certification Bodies to carry out Product Marking Verification, Production Control Audits and CE Marking Certification against the following EU Directives:
Chemicals
- Chemical substances (REACH)
- Explosives for civil uses
- Pyrotechnic articles
Conformity assessment and management systems
- New Legislative Framework (NLF) and Eco-Management and Audit Scheme (EMAS)
Construction
- Construction products (CPD/CPR)
Consumers and workers protection
- Cosmetics products
- General product safety
- Personal protective equipment (PPE)
- Toys safety
Energy efficiency
- Ecodesign and energy labelling
Electric and electronic engineering
- Electromagnetic compatibility (EMC)
- Equipment for explosive atmospheres (ATEX)
- Low Voltage (LVD)
- Radio Equipment (RED)
- Restriction of the use of certain hazardous substances (RoHS)
Healthcare engineering
- Active implantable medical devices
- In vitro diagnostic medical devices
- Medical devices (MDD)
Measuring technology
- Measuring instruments (MID)
- Non-automatic weighing instruments (NAWI)
Mechanical engineering and means of transport
- Cableway installations designed to carry persons
- Equipment for explosive atmospheres (ATEX)
- Gas appliances (GAR)
- Inspection of pesticide application equipment
- Lifts
- Machinery (MD)
- Pressure equipment (PED)
- Rail system: interoperability
- Recreational craft and personal watercraft
- Simple Pressure Vessels (SPVD)
- Unmanned aircraft systems (UAS)
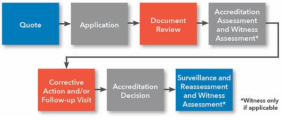
Please refer to the information about the accreditation process:
British Accreditation Registrars’ Accreditation for Inspection Agencies”
- Demonstrates compliance with CE Marking Accreditation.
- BAR offers prompt, personal service, including rapid scheduling of assessments to meet the needs of inspection agencies.
- Accreditation serves as an internationally recognized “stamp of approval” for industry and regulators.
- Accreditation increases the recognition and acceptance of inspection reports across domestic and national borders.
- Accreditation helps to reduce costs for manufacturers and exporters by reducing or eliminating the need for re-inspecting in another economy.
Certification Bodies interested to apply for Accreditation for GMP can send an email to info@bar-registrars.org demanding for following Application Practice
- General Criteria for Accreditation Requirements
- General Criteria for Conditions for the Use of BAR Accreditation Symbol
- Applicable Fees
- Documentation Checklist as per CE Marking
- BAR Transition Policy for ISO 17021-1:2015
Accreditation Criteria :
- ISO 17021 – Requirements for Bodies providing audit and certification of management systems.
- IAF MD 1:2007 – Certification of Multiple Sites Based on Sampling.
- IAF MD 2:2007 – Transfer of Accredited Certification of Management Systems.
- IAF MD 3:2008 – Advanced Surveillance and Recertification Procedures.
- IAF MD 4:2008 – Use of Computer Assisted Auditing Techniques for Accredited Certification of Management Systems.
- IAF MD 5:2013 – IAF Mandatory document for duration of QMS & EMS Audits.
- IAF MD 11:2013 – IAF Mandatory Document for the application of ISO 17021 for audits of Integrated Management Systems.



