ISO 13485
Accreditation for ISO 13485 (Quality Management System-Medical Devices)
British Accreditation Registrars accredits Certification Bodies for compliance against ISO 1385:2016 through verification of their existing ISO 17021 system. The accreditation is done against Scheme No- 30209.
ISO 13485:2016 Accreditation for a Certification body (CBs) helps them to audit and provide accreditated Certification to their Clients.
ISO 13485 is the Quality Management System standard for Medical Devices and helps to demonstrate the effective solution to meet the comprehensive requirements for a QMS in the medical device industry. The standard enables organizations to establish the systems and processes necessary to improve and update the quality as per new technology and techniques to cater to customer requirements and needs.
ISO 13485 requirements cover-up all the Quality Policy, Quality Objectives, and Quality Manual for implementation of the Quality Management System. Adopting ISO 13485 provides a practical foundation for manufacturers to address the EU Medical Device Directive (MDD), the EU Medical Device Regulation (MDR), and other regulations, as well as demonstrating a commitment to safety and quality of medical devices for their users.
A Certification Body applying for ISO 13485 accreditation must conform to ISO/IEC 17021 and other additional International requirements as detailed in Specific Requirements for Accreditation for QMS-MD Scheme.
Benefits of Accreditated Certification of ISO 13485 Certification
- Businesses perceive accredited certification as providing value for money
- Updated use of technology
- Improved operational efficiencies and maintenance practices
- Protecting and improvement in risk management
- Customer satisfaction that improves client retention
- Achieve International approval
- Deliver value-added outcomes
- Leading independent market research company
- Employers often require evidence that the Certificate that they have received is from an Accredited Body
- Enjoy Competitor Edge
Please refer to the information about the accreditation process:
British Accreditation Registrars’ Accreditation for Inspection Agencies”
- Demonstrates compliance with ISO 13485 Accreditation.
- BAR offers prompt, personal service, including rapid scheduling of assessments to meet the needs of inspection agencies.
- Accreditation serves as an internationally recognized “stamp of approval” for industry and regulators.
- Accreditation increases the recognition and acceptance of inspection reports across domestic and national borders.
- Accreditation helps to reduce costs for manufacturers and exporters by reducing or eliminating the need for re-inspecting in another economy.
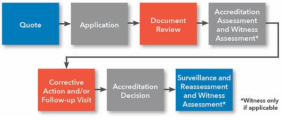
Certification Bodies interested to apply for Accreditation for ISO 13485 can send an email to info@bar-registrars.org demanding for following Application documents.
- Accreditation Application for Quality Management System for Medical Devices
- General Criteria for Accreditation Requirements
- General Criteria for Conditions for the Use of BAR Accreditation Symbol
- Applicable Fees
- Documentation Checklist as per QMS-MD
- BAR Transition Policy for ISO 17021-1:2015
Accreditation Criteria :
- ISO 17021 – Requirements for Bodies providing audit and certification of management systems.
- IAF MD 1:2007 – Certification of Multiple Sites Based on Sampling.
- IAF MD 2:2007 – Transfer of Accredited Certification of Management Systems.
- IAF MD 3:2008 – Advanced Surveillance and Recertification Procedures.
- IAF MD 4:2008 – Use of Computer Assisted Auditing Techniques for Accredited Certification of Management Systems.
- IAF MD 5:2013 – IAF Mandatory document for duration of QMS & EMS Audits.
- IAF MD 11:2013 – IAF Mandatory Document for the application of ISO 17021 for audits of Integrated Management Systems.



