GMP
Accreditation for GMP (Good Manufacturing Practice)
British Accreditation Registrars accredits Certification Bodies for compliance against GMP through verification of their existing ISO 17021 system. The accreditation is done against Scheme No- 22001.
GMP Accreditation for a Certification body (CBs) helps them to audit and provide accreditated Certification to their Clients.
GMP is the Good Manufacturing Practice also referred to as cGMP helps to that ensures that medicinal products are consistently produced and controlled to the quality standards. It helps companies to minimize or eliminate instances of contamination, mix-ups, and errors for pharmaceuticals or drugs, active pharmaceutical ingredients, diagnostics, foods, and medical devices.
Whilst there is no formal mandatory requirement to comply with cGMP during early clinical development in phase I and phase II Clinical Trials, cGMP MUST be fully implemented by phase III Clinical Trials and for all subsequent manufacturing activities.
A Certification Body applying for GMP accreditation must conform to ISO/IEC 17021 and other additional International requirements as detailed in Specific Requirements for Accreditation for GMP Scheme.
Benefits of Accreditated Certification of GMP Certification
- Businesses perceive accredited certification as providing value for money
- Exceeds minimum standards of quality
- Process and analytical validation
- Providing assurance for the quality
- Achieve International approval
- Deliver value-added outcomes
- Leading independent market research company
- Employers often require evidence that the Certificate that they have received is from an Accredited Body
- Enjoy Competitor Edge
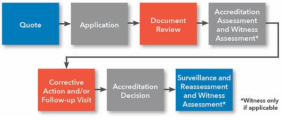
Please refer to the information about the accreditation process:
British Accreditation Registrars’ Accreditation for Inspection Agencies”
- Demonstrates compliance with GMP Accreditation.
- BAR offers prompt, personal service, including rapid scheduling of assessments to meet the needs of inspection agencies.
- Accreditation serves as an internationally recognized “stamp of approval” for industry and regulators.
- Accreditation increases the recognition and acceptance of inspection reports across domestic and national borders.
- Accreditation helps to reduce costs for manufacturers and exporters by reducing or eliminating the need for re-inspecting in another economy.
Certification Bodies interested to apply for Accreditation for GMP can send an email to info@bar-registrars.org demanding for following Application Practice
- General Criteria for Accreditation Requirements
- General Criteria for Conditions for the Use of BAR Accreditation Symbol
- Applicable Fees
- Documentation Checklist as per GMP
- BAR Transition Policy for ISO 17021-1:2015
Accreditation Criteria :
- ISO 17021 – Requirements for Bodies providing audit and certification of management systems.
- IAF MD 1:2007 – Certification of Multiple Sites Based on Sampling.
- IAF MD 2:2007 – Transfer of Accredited Certification of Management Systems.
- IAF MD 3:2008 – Advanced Surveillance and Recertification Procedures.
- IAF MD 4:2008 – Use of Computer Assisted Auditing Techniques for Accredited Certification of Management Systems.
- IAF MD 5:2013 – IAF Mandatory document for duration of QMS & EMS Audits.
- IAF MD 11:2013 – IAF Mandatory Document for the application of ISO 17021 for audits of Integrated Management Systems.



